The Australian Government has answered the prayers of a Balaklava family desperate to save their little girl.
Seven-year-old Holly Zerk, as well as around 20 other Australian children suffering from high-risk neuroblastoma, have been given a lifeline, with Federal Health Minister, Mark Buttler, giving them free access to DFMO, a groundbreaking treatment known not otherwise available in Australia.
The medicine was approved by the medicines regulator in the United States, the Food and Drug Administration, in December 2023 and has been shown to reduce the risk of relapse and improve survival of patients suffering high-risk neuroblastoma.
While some families have sought treatment overseas in the U.S., costing them up to $500,000, other children have missed out because they couldn’t afford the treatment or were too sick to travel.
Holly Zerk’s family made a plea to the Federal Health Minister last week, to “be the hero these kids need”.
After bravely enduring five rounds of chemotherapy, twelve sessions of radiation, two high-dose chemotherapy rounds with stem cell transplants, and four rounds of immunotherapy, the farming family were facing the daunting prospect of relocating Holly to the US for two years to undergo DFMO.
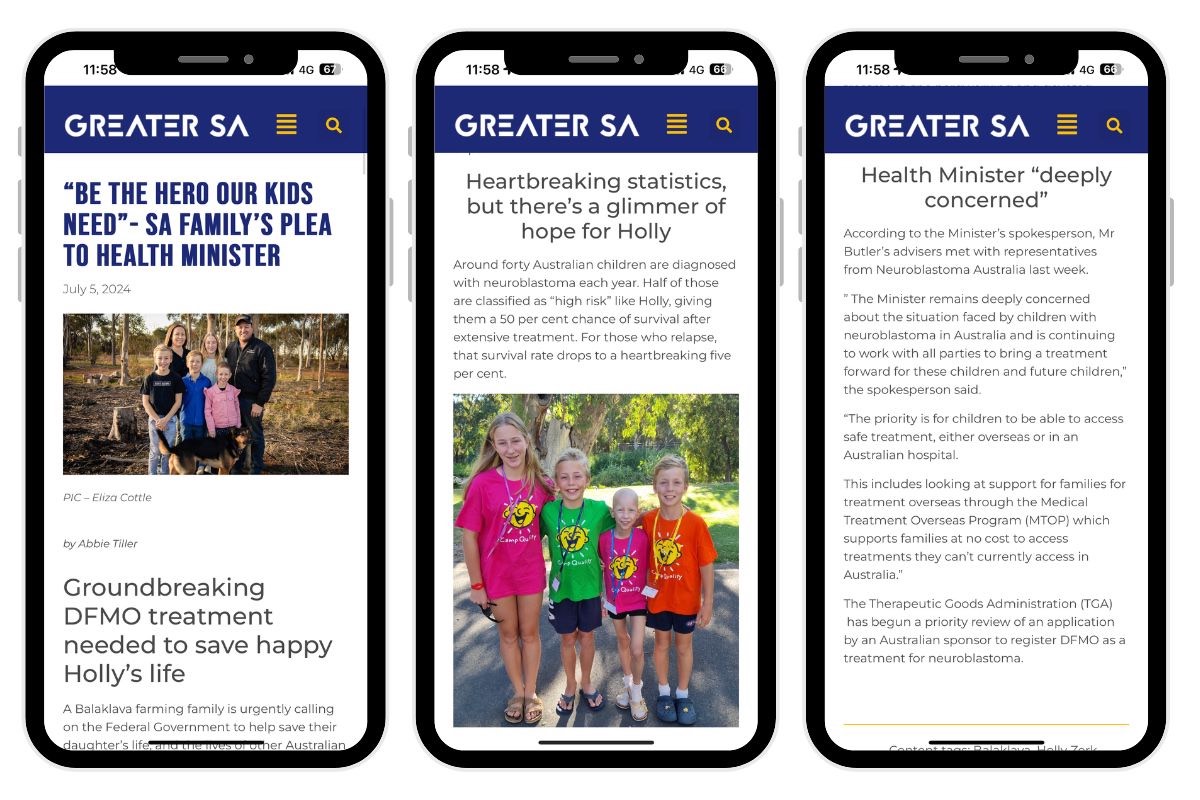
“I’m happy I can just do my treatment in Australia now and I can go to school and netball and dance instead of heaps of plane rides,” brave little Holly said.
For Mum and Dad, Travis and Lee-Anne, a stressful load has been lifted from their shoulders and so many other families. “We are just so thankful to Mark Butler for providing the use of DFMO in Australia,” Lee-Anne said.
“We feel so relieved it’s one less thing to plan the next two years of our life around. Thank you.”
The Commonwealth will provide funding to state and territory governments to support them in administering DFMO to eligible patients, free of charge, through the Drug and Therapeutics Committees of major public hospitals, while it pursues the necessary approvals for a PBS listing.
“We understand that DFMO offers the only hope to some patients who are desperately ill from neuroblastoma,” Minister Butler said.
“This one-off funding ensures that they can get this new and promising treatment, without the huge price tag, while proper approval processes are followed.
“I am delighted that the company sponsoring the medicine for use in Australia is also looking to establish a compassionate access scheme to provide DFMO to those patients in need.
“In the coming days, I will be writing to my state and territory health colleagues to ask them to consider providing DFMO on the basis of the Government’s commitment to reimburse them for the cost of purchasing this medicine.”







